Have you ever wondered where metals hide on the periodic table? If you’re curious about how to spot metals quickly and why their position matters, you’re in the right place.
Understanding which side of the periodic table metals occupy can make your study of chemistry clearer and more exciting. Keep reading, and you’ll discover simple tips to identify metals at a glance—and why their location shapes their unique properties. This knowledge will not only boost your confidence but also make chemistry feel a lot less confusing.
Ready to unlock the secret? Let’s dive in!

Credit: elementsandtheperiodictable.weebly.com
Metal Location On The Table
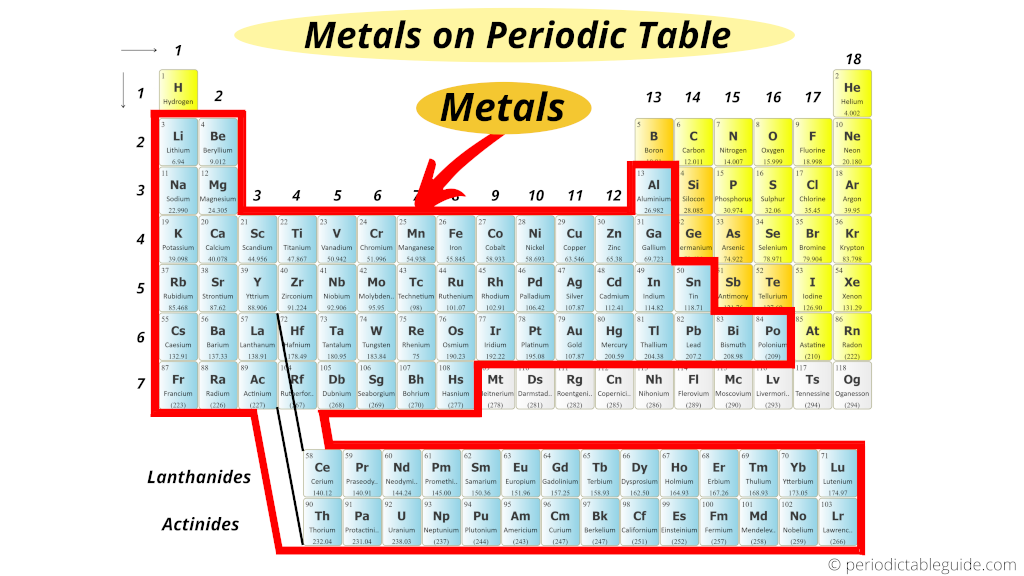
The periodic table organizes all known elements in a clear, structured way. Metals take up a large part of this table. Understanding their exact location helps to grasp their common features and uses. The placement of metals also shows how they relate to other types of elements.
Left Side Dominance
Most metals are found on the left side of the periodic table. These include alkali metals and alkaline earth metals. They are highly reactive and good conductors of electricity. These metals are soft and often shiny. Their position shows they easily lose electrons in reactions.
Transition Metals In The Center
The center block of the table contains transition metals. These metals are harder and less reactive than those on the left. They can form different compounds and colors. Transition metals are useful in construction and electronics. Their location reflects their unique properties and versatility.
Metalloids As Boundary Markers
Metalloids sit between metals and nonmetals. They act as a boundary on the periodic table. These elements have mixed properties of metals and nonmetals. Their placement helps separate the metallic elements from the nonmetallic ones. Metalloids are important in semiconductors and technology.
Characteristics Of Metals
Metals make up a large part of the periodic table. They share certain traits that set them apart from other elements. Understanding these traits helps us know why metals behave the way they do. It also explains their many uses in daily life and industry.
Physical Properties
Metals are usually shiny and have a metallic luster. They are good conductors of heat and electricity. Most metals are solid at room temperature, except mercury. Metals can be bent or hammered without breaking. This quality is called malleability. They also stretch into wires, known as ductility. Metals tend to have high melting and boiling points.
Chemical Behavior
Metals often lose electrons during chemical reactions. This makes them form positive ions or cations. They react with oxygen to form oxides. These oxides are usually basic in nature. Metals also react with acids, producing hydrogen gas. Their chemical activity varies from very reactive to less reactive metals.
Common Uses
Metals are essential in construction and manufacturing. Iron and steel build bridges, buildings, and cars. Copper is used in electrical wiring due to its conductivity. Aluminum is popular in packaging and aircraft. Gold and silver serve in jewelry and electronics. Metals also play roles in tools, machinery, and coins.
Nonmetals And Their Placement
Nonmetals are important elements in the periodic table. They have unique properties that set them apart from metals. Nonmetals mostly appear on the right side of the periodic table. Their placement helps us understand their behavior and uses.
Right Side Elements
Nonmetals are found on the right side of the periodic table. This includes elements like oxygen, nitrogen, and chlorine. They are located opposite to metals, which are mostly on the left side. The right side placement shows their tendency to gain electrons. This helps them form negative ions or share electrons in bonds.
These elements are essential for life and many chemical processes. For example, oxygen supports breathing, and nitrogen is a key part of proteins. Their position on the right side also means they have higher electronegativity. This means they attract electrons more strongly than metals.
Differences From Metals
Nonmetals differ from metals in many ways. They are usually poor conductors of heat and electricity. Metals conduct heat and electricity well. Nonmetals are often brittle or gaseous, unlike the solid and malleable metals. Nonmetals do not shine like metals do. Instead, they have dull appearances.
Nonmetals form acidic oxides, while metals form basic oxides. Their chemical reactions also differ. Nonmetals tend to gain or share electrons, while metals lose electrons. These differences explain why metals and nonmetals have distinct roles in chemistry and daily life.
Credit: periodictableguide.com
Metalloids Between Metals And Nonmetals
Metalloids sit between metals and nonmetals on the periodic table. They share traits from both groups. This unique position makes them interesting in chemistry and technology. They do not fit fully as metals or nonmetals.
Mixed Properties
Metalloids show mixed properties. They can conduct electricity but not as well as metals. Some are shiny like metals but brittle like nonmetals. Their chemical behavior varies depending on the elements they react with. This mix allows metalloids to be useful in many areas.
Position And Role
Metalloids are located along the zigzag line on the periodic table. This line divides metals on the left and nonmetals on the right. Their position helps them act as a bridge between these two groups. Many metalloids are important in electronics and glassmaking. They play a key role in modern materials.
Periodic Table Trends
The periodic table shows clear trends in the properties of elements. These trends help us understand where metals are located. By studying these patterns, we see how metallic character changes across the table. This knowledge is key to grasping why metals appear mostly on one side.
Periodic table trends reveal how metals behave as you move across periods and down groups. The changes in atomic structure influence metallic traits. These trends explain the placement of metals mainly on the left side and center of the table.
Metallic Character Across Periods
As you move from left to right across a period, metallic character decreases. Elements on the left have a strong metallic nature. They easily lose electrons and conduct electricity well.
On the right side, elements tend to be nonmetals. They gain or share electrons instead of losing them. This shift happens because atoms gain more protons, pulling electrons closer.
This pull makes atoms less likely to lose electrons, reducing metallic traits. So, metals mostly appear on the left side of each period.
Metallic Character Down Groups
Moving down a group, metallic character increases. Atoms get bigger and outer electrons are farther from the nucleus. This makes electrons easier to lose.
Because of this, elements lower in a group show stronger metallic properties. They are more reactive and conduct electricity better.
This trend explains why heavier metals are found at the bottom of each group. The increase in metallic character down groups complements the decrease across periods.
Exceptions And Special Cases
The periodic table mostly groups metals on its left side. Still, a few elements do not fit neatly into this pattern. These exceptions and special cases show how diverse elements can be. Understanding them helps us see the full picture of the table.
Hydrogen’s Unique Spot
Hydrogen sits at the top left of the periodic table. It looks like a metal because of its position. But it is actually a non-metal gas. It can behave like metals or non-metals in different conditions. This makes hydrogen very special and hard to classify.
Post-transition Metals
Post-transition metals appear just to the right of the transition metals. These include elements like tin and lead. They have some metal properties but are softer and less reactive. Their position shows they share traits with both metals and non-metals. These metals often have unique uses because of their mixed characteristics.

Credit: www.youtube.com
Frequently Asked Questions
Which Side Of The Periodic Table Contains Metals?
Metals are primarily located on the left and center of the periodic table. They occupy groups 1 to 12 and some in groups 13 to 16. This arrangement reflects their shared metallic properties like conductivity and malleability.
Are Transition Metals In The Middle Of The Table?
Yes, transition metals are situated in the middle section, specifically groups 3 to 12. They exhibit unique properties such as variable oxidation states and are good conductors of heat and electricity.
Where Are Alkali Metals Found On The Table?
Alkali metals are located on the far left side of the periodic table in group 1. They are highly reactive and include elements like lithium, sodium, and potassium.
Do Metals Appear On The Right Side Of The Periodic Table?
Generally, metals are not on the right side; that area mostly contains nonmetals and noble gases. Metals dominate the left and center sections of the table.
Conclusion
Metals mainly sit on the left side of the periodic table. They show traits like shine, strength, and good heat or electricity flow. Knowing where metals are helps us understand their uses better. It also makes learning science easier and more fun.
Keep exploring the periodic table to see how elements fit and work together. The table tells a story about the building blocks of everything around us.
As an Amazon Associate, I earn from qualifying purchases.