Have you ever wondered why some elements on the periodic table react quickly, while others barely change? Understanding which side of the periodic table is more reactive can unlock the secrets behind everyday chemical reactions.
Whether you’re curious about why metals rust or how fireworks light up the sky, knowing where reactivity lies will give you a clearer picture of the world around you. Keep reading, and you’ll discover surprising facts that make chemistry easier—and more exciting—than you ever imagined.
Reactivity Trends Across The Table
The periodic table shows how elements react based on their position. Reactivity changes across rows and columns. Elements on different sides behave very differently. Understanding these trends helps explain which side is more reactive.
Reactivity depends on how easily atoms lose or gain electrons. This varies for metals and nonmetals. The table groups elements with similar properties together. Both group and period affect reactivity strongly.
Reactivity Of Metals Vs Nonmetals
Metals are mostly on the left side of the table. They lose electrons easily and react quickly. Alkali metals, in group 1, are very reactive. Their atoms have one electron to give away.
Nonmetals, on the right side, gain electrons to react. They do not lose electrons easily. Halogens, in group 17, are very reactive nonmetals. They need one electron to complete their outer shell.
Metals react by losing electrons; nonmetals react by gaining. This difference creates opposite trends in reactivity.
Group And Period Influence
Reactivity changes down a group because atoms grow bigger. For metals, bigger atoms lose electrons more easily. This makes metals more reactive down the group.
Nonmetals become less reactive down a group. Larger atoms hold electrons less tightly. They do not attract electrons as well.
Across a period, reactivity changes too. Metals become less reactive moving right. Nonmetals become more reactive moving left to right.
This pattern forms because atoms gain more protons across a period. More protons pull electrons closer. This affects how atoms react.

Credit: www.youtube.com
Left Side Elements
The left side of the periodic table contains some of the most reactive elements. These are metals that easily lose electrons. They form positive ions quickly. Their reactivity is a key reason for their importance in chemistry and industry.
This side mainly includes alkali metals and alkaline earth metals. Both groups show strong reactivity but differ in how fast they react. Understanding these differences helps explain many chemical behaviors.
Characteristics Of Alkali Metals
Alkali metals are in Group 1 of the periodic table. They have one electron in their outer shell. This electron is easily lost in chemical reactions. Losing it forms a +1 charge ion.
These metals are soft and shiny. They have low melting points compared to other metals. Alkali metals react violently with water. This reaction produces hydrogen gas and a strong base.
Reactivity increases as you move down the group. Lithium is the least reactive, while cesium is very reactive. Their high reactivity limits their natural occurrence in pure form.
Reactivity Patterns In Alkaline Earth Metals
Alkaline earth metals belong to Group 2. They have two electrons in their outer shell. These metals lose both electrons to form +2 charge ions.
They are harder and denser than alkali metals. Their melting points are higher as well. These metals react with water, but less violently than alkali metals.
Reactivity also increases down the group. Beryllium reacts very slowly, while radium is highly reactive. Their chemical behavior is important for many industrial processes.
Right Side Elements
The right side of the periodic table holds some unique elements. These elements include the halogens and noble gases. Their chemical behaviors differ greatly. Some are very reactive, while others are very stable. Understanding their properties helps explain their roles in chemistry and daily life.
Halogens And Their High Reactivity
Halogens are found in Group 17 of the periodic table. They include fluorine, chlorine, bromine, iodine, and astatine. These elements are very reactive. They tend to gain one electron to fill their outer shell. This makes them eager to react with other elements. Halogens often form salts when combined with metals. Their reactivity decreases down the group. Fluorine is the most reactive, while iodine is less so. This reactivity makes halogens useful in disinfectants and water treatment.
Noble Gases And Their Stability
Noble gases are in Group 18. They include helium, neon, argon, krypton, xenon, and radon. These gases have full outer electron shells. This makes them very stable and mostly unreactive. They rarely form compounds with other elements. Their stability is why they are called “noble.” Noble gases are used in lighting and balloons. Their low reactivity means they do not corrode or react easily. This stability contrasts sharply with the halogens on the same side.
Credit: www.scienceabc.com
Factors Affecting Reactivity
Reactivity of elements on the periodic table depends on several key factors. These factors affect how easily an atom loses or gains electrons. This, in turn, influences the element’s chemical behavior and its position on the table. Understanding these factors helps explain why one side of the table is more reactive than the other.
Atomic Size And Ionization Energy
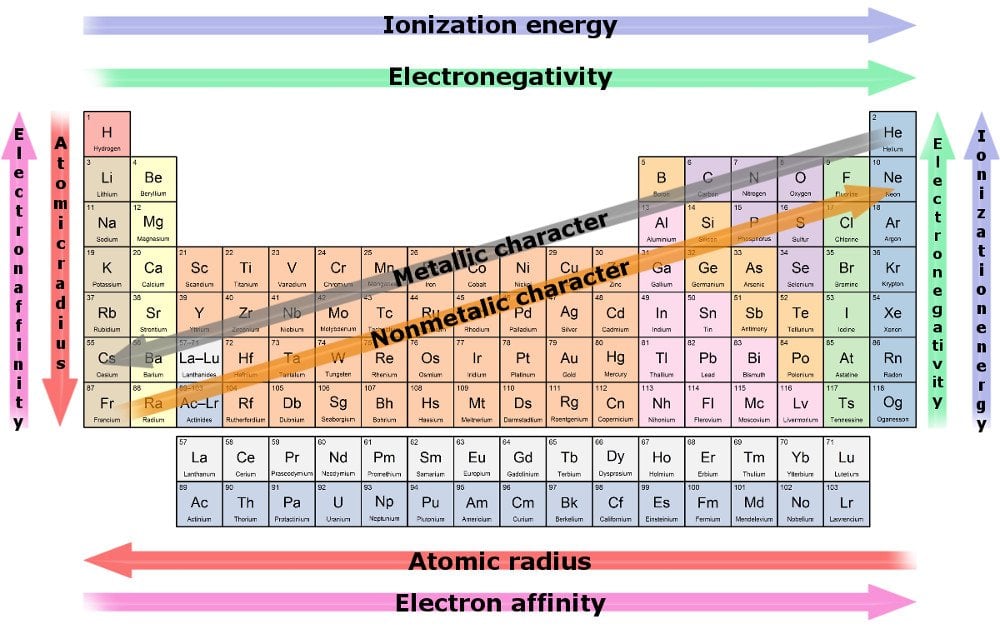
Atomic size is the distance from the nucleus to the outermost electrons. Larger atoms have electrons farther from the nucleus. This makes it easier for the atom to lose electrons and react. Ionization energy is the energy needed to remove an electron. Atoms with low ionization energy lose electrons easily. On the left side of the periodic table, atoms are larger and have lower ionization energy. This makes them more reactive, especially the alkali metals.
Electron Affinity And Electronegativity
Electron affinity shows how much an atom wants to gain electrons. Atoms with high electron affinity gain electrons quickly. Electronegativity measures how strongly an atom pulls electrons in a bond. Atoms on the right side have higher electron affinity and electronegativity. This makes them more reactive in gaining electrons. Halogens, found on the right, are very reactive because they want to fill their outer shells.
Unexpected Reactivity Cases
Reactivity on the periodic table is not always clear-cut. Elements do not always behave as expected based on their position. Some groups show surprising variations in how they react. These unexpected cases reveal the complexity of chemistry. They challenge the idea that reactivity increases smoothly across the table.
Transition Metals’ Variable Reactivity
Transition metals sit in the center of the periodic table. They show a wide range of reactivity levels. Some, like iron and copper, react slowly or only under certain conditions. Others, such as platinum, hardly react at all. Their electrons in d-orbitals create unique bonding possibilities. This makes their chemical behavior less predictable. Their reactivity depends on the element’s oxidation state and environment.
Metalloids And Their Dual Behavior
Metalloids have properties between metals and nonmetals. They can act as either, depending on the situation. For example, silicon behaves like a metal in some reactions and a nonmetal in others. This dual nature causes varied reactivity patterns. Metalloids often form compounds with both metallic and nonmetallic elements. Their position on the periodic table shows this mixed behavior clearly.
Practical Implications
Understanding which side of the periodic table is more reactive has clear practical uses. Reactivity affects how elements behave in real-world settings. This knowledge helps industries choose the right materials. It also guides safe handling and storage of chemicals. Below are key practical points to consider.
Industrial Applications Of Reactive Elements
Elements on the left side of the periodic table, like alkali metals, are highly reactive. Industries use them in many ways. For example, sodium and potassium help in making fertilizers and soaps.
On the right side, halogens are also reactive. They are useful in water purification and making disinfectants. Their strong reactivity kills bacteria effectively.
Knowing reactivity helps manufacturers create better products. It allows them to control reactions for desired results. This knowledge saves time and reduces costs.
Safety Considerations With Highly Reactive Elements
Highly reactive elements can be dangerous. They often react violently with water or air. This can cause fires or explosions.
Proper storage is vital. Reactive metals need to be kept in oil or sealed containers. Halogens require cool, dry places.
Workers must use protective gear and follow strict protocols. Safety training reduces accidents and health risks. Understanding reactivity helps prevent harm in labs and factories.

Credit: chemed.chem.purdue.edu
Frequently Asked Questions
Which Side Of The Periodic Table Is More Reactive?
The left side of the periodic table, especially alkali metals, is more reactive. These elements easily lose electrons to form positive ions. Reactivity decreases moving right across a period and increases down a group on the left side.
Why Are Alkali Metals Highly Reactive?
Alkali metals are highly reactive because they have one valence electron. This electron is easily lost, forming positive ions. Their low ionization energy makes them eager to react with other elements, especially nonmetals like halogens.
How Does Reactivity Vary Across The Periodic Table?
Reactivity decreases from left to right across a period due to increasing nuclear charge. It increases down a group as atomic size grows, making it easier to lose or gain electrons. Metals react more on the left; nonmetals on the right.
Are Nonmetals On The Right Side Reactive?
Yes, nonmetals on the right side, especially halogens, are highly reactive. They gain electrons easily to form negative ions. Their high electronegativity drives vigorous reactions, especially with metals from the left side of the table.
Conclusion
The left side of the periodic table is the most reactive. Elements here easily lose electrons. This makes them very active in chemical reactions. The right side, especially the noble gases, is less reactive. They do not lose or gain electrons easily.
Understanding this helps explain many chemistry facts. It shows why metals and nonmetals behave differently. Chemistry becomes clearer with this knowledge. Reactivity depends on electron behavior and element position. Simple, yet important to remember.
As an Amazon Associate, I earn from qualifying purchases.